“I have never let my schooling interfere with my education” ~Mark Twain
I have an undergraduate degree in chemistry. I got 90’s in my organic chemistry course so I feel qualified to explain this. I didn’t care so much for the other fields of chemistry at the time. I like having the freedom to study what I want to study and I am not. I would balance my 90 with 60’s to get a 70 average. My respect goes to those with the discipline to do well in the sciences.
I assume you have no prior knowledge but are interested in science. If anything is not clear just leave a comment and I’ll try to help you out the best I can.
Considering the popularity of the TV show Breaking Bad, organic chemistry is the branch of chemistry most people will ask about. Although illicit drug manufacture is a fascinating issue we must start with the basic principles of the science. In theory we have access to all reagents with no need for consideration of danger, legality and ethics. Only after we understand the basic premise can we discuss practical matters with real life limitations.
Basic Chemistry
Let us begin with the most emblematic table of the science.
We will only deal with the first ten elements in this discussion, from hydrogen to neon so don’t worry about it too much. The lighter (smaller numbered) elements are much more common than the heavier elements in the universe and form the basis of organic chemistry.
The number refers to how many protons are in the nucleus of the element. If you change the number of protons you change the element itself. As chemists we can not change an element, only the bonds between elements. Protons carry a positive charge (+) that are canceled out by the negative charge (-) electrons carry. Electrons are found orbiting the nucleus. Electrons are much smaller and much more mobile than the nucleus. As chemists we manipulate electrons to connect these unchangeable nuclei together. Note that hydrogen, represented by H has 1 proton. The term proton is used interchangeably with hydrogen when dealing with acids.

We also have neutrons but they are not important right now. They have no charge and are found with the protons.
A solution that carries a positive charge is known as an acid. A solution with a negative charge is knowing as a base. Consider mixing baking soda (weak base) with vinegar (weak acid). Vinegar and baking soda are both stable compounds and can be stored on the shelf. It takes a certain amount of energy to initiate a change, if this amount of energy is reached the reaction will proceed because it will go to a lower energy state, releasing energy in the process which makes the reaction go even faster.

Nature tries to balance energy by seeking out the lowest energy state. You happen to go down a hill easily, yet climbing it takes effort. A chemical bond represents a energetic valley in which energy must be applied to change the arrangement. The deeper the valley the more energy is required for change to happen. This energy comes from heat usually. Substances in deep valleys will be heat resistant and stable, while a substance in a shallow valleys on a mountain are explosives. No substance exists on a peak for any appreciable amount of time and can not be collected.
The more energy we need to put into our components to get them over the hump the more stable they will be. Stability is serious practical consideration because unstable compounds may burn if they contact water,and more unstable compounds may explode on contact with air even. On the other hand things only go down hill spontaneously, so the higher energy we can get into reactants the more possible reactions will be available to us. We want to stand on energetic high ground. However the higher the energy, the greater the expense and difficulty. We want accesses to the strongest reagents to be able to build more substances and we want to use the least amount of energy we use for practical reasons. A reaction needed to be carried under an inert atmosphere at -72ºC is much harder to do than one done at room temperature on the counter for example.
Organic Chemistry
Organic chemistry is the chemistry of carbon. It should come as no surprise carbon is special when we consider the racket about global warming and carbon credits while no such mention is made of the other elements. Carbon is unique because it forms stable bonds with itself and virtually any other element. Thus it is romantically termed the “back bone of life”. This is an apt analogy seen in the way we represent chemical structure in symbols.

This is ethanol. This is what you drink to get drunk. This is its molecular structure represented. Typically, since carbon is so common, the C is not written and is implied at every unlabeled vertices (like the points on the zig zag line pattern. Very often H is not even written in and is implied by how many lines are coming out of C. A rule is that carbon always has 4 lines coming out of it, Oxygen has 2 lines, hydrogen 1 line. Any deviation from this rule will be marked with a + or – sign and is easy to spot indicating if its acidic or basic in solution or it is termed a salt if it is solid.
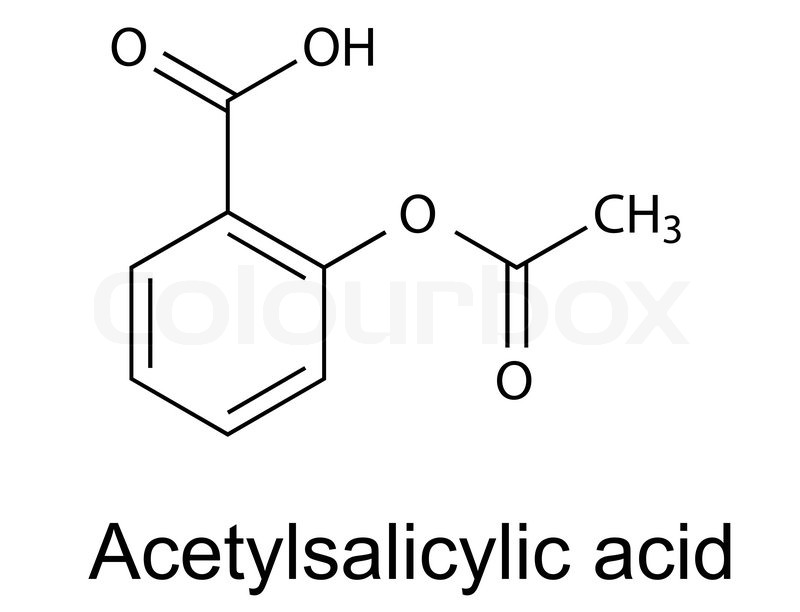
AKA aspirin. The carbons are any point. The double lines indicate a double bond. The bottom left most carbons in the ring each have a H that isn’t drawn in to give them 4 bonds. Organic chemistry is a pictorial language and study is required to become fluent in it. The elements are the alphabet, drugs are the sentences and functional groups are the words. Functional groups are the common arrangements of elements and let us determine the important features. When making a molecule we want to look at it in terms of functional groups. Six sided rings with three double bonds are a common motive and are very stable. If what we want to make contains a 6 sided ring with 3 double bonds we will need to find them ready made until we are skilled chemists.

Here is a basic reaction and how we would go about synthesizing aspirin. We mix acetic anhydride and salicylic acid in a flask, add a small amount of phosphoric acid and heat it. In practice exact proportions and times need to be figured out.

^Acetic anhydride also written as (CH3CO)2O sometimes.
To learn from this reaction so we can apply the principle to other reactions we must determine how this reaction happens and if we expect to get any other products A yield of 70% of the theoretical maximum is considered decent. Maximizing yields improves profitability and is a branch of research in itself. We must always be aware of what is made as a by product in pursuit of our goal. 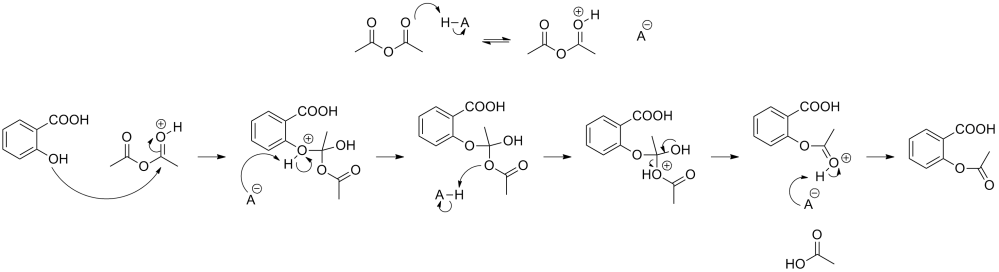
A proposed mechanism of the reaction. Determining if a proposed mechanism is the correct mechanism is the aim of many clever experiments. First we must learn what is going on.
The top says the acid is destabilizing the acetic anhydride by absorbing its electrons, leaving it with a positive charge. This effectively pushes it up the valley in our energy diagram. This accounts for the reason we need to add an acid in the first place.
The OH group attacks the carbon connected to the destabilized oxygen and is ejected a vinegar. This is illustrated in a few steps, determining that level of detail is very difficult for more complicated reactions. The arrows show where electrons or negative charge is flowing. To understand this fully will take a respectable amount of study, but this gives you a good idea of what to look forward to.
If we wanted to make aspirin we would need to look into the exact equipment we need and the price of materials we will need. Also we need to consider other ways of making aspirin, such as extracting it from willow bark. How will we meet licensing regulations etc. etc.?
Social Issues
Some people assume that because something is natural it is “better” than something synthetic. This is mainly due to marketing playing on people’s natural fears of what they do not understand. Many life saving substances are not found in nature and must be made synthetically. Some are found naturally but in too small concentrations to be effectively collected. An example would be the hormones used to induce women into labour. For these to be available for general use it must be made synthetically. A synthetic approach must be used often times.
In our example of aspirin, extraction from the plant is only practical on small scales. It is only possible to be so cheap and readily available because the artificial synthesis is so easy, using readily available materials. Massive willow farms would be needed to supply it with a natural method on a worldwide scale.
There is some concern about synthetic methods that is valid. Many reactions use highly poisonous substances such as chromate. A clean substance can be obtained, but great care must be taken to ensure purification of product and disposal of waste. An ignorant chemist might not bother with purification or do it incorrectly. This can lead to health complications many years in the future for consumers of tainted product.
Also in some cases a natural method produces a better product. Synthetic vanilla can be made and is chemically identical to the major component of natural vanilla. However natural vanilla also includes many related compounds which add to the taste and aroma. Some products are pleasing due to a complicated blend of compounds that would be impractical to make and blend in an ideal proportion. It might be better to grow healthy plants instead.
Do not be fooled by the “natural” marketing schtick, for some things synthetic is better and some things natural is better. It may change depending on the scale of the product too. It needs to be decided on a case by case basis.
I saw in A&W the other day beef raised without use of hormones or steroids used. While good advertising, is technically incorrect. Cows use steroids and hormones to regulate biological processes, the word artificial is needed in the claim.
The fear of scientific achievements runs deep and creates negative outcomes for society when these people try to decide on matters concerning public health such as people against vaccinations. This fear of vaccinations is now a public health risk. These people are ignorant of the havoc diseases that modern medicine holds off. Scientific education is deplorable considering the importance it plays in modern living. I feel high schools should aim at developing scientifically literate students, at least for the university tack here (Canadian system). Consider that if we abolish vaccines we would have a catastrophe that would make WW III a joke. Historically disease has played a much larger role in warfare than armies battling one another. Bacteria has killed more people than bullets. To refuse such a boon like vaccines for society is retarded, it is shocking at how prevalent it is to see people who think otherwise.
I hope this article has made you think more deeply about scientific matters and made you less prone to inaccurate thinking. If you would like to see more scientifically oriented articles from me please share this with interested people. Thanks for reading!
(I figured out tags so I can judge how popular my subjects are)
.svg/2000px-Periodic_table_(polyatomic).svg.png)
weiner
LikeLike
buns and ketchup
LikeLike